Why membrane chromatography may be beneficial to your protein purification process. Why and where you should use membrane chromatography.
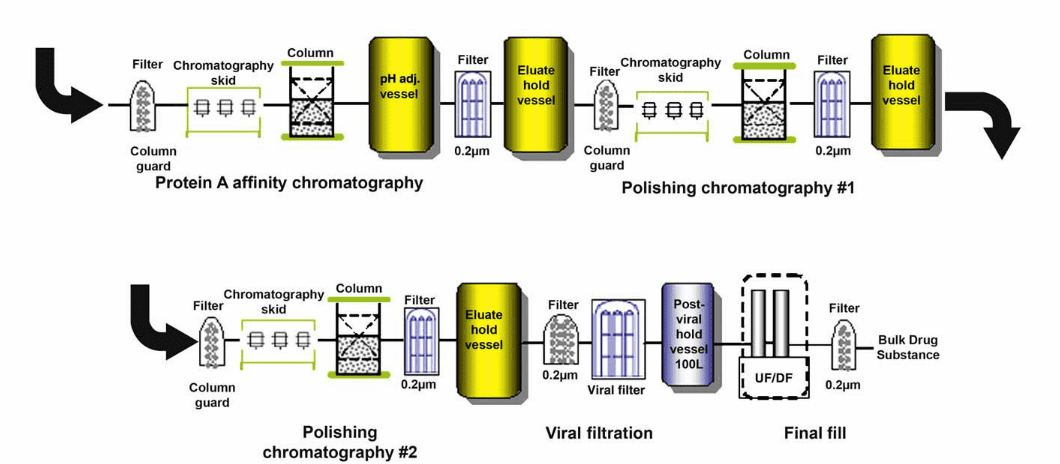
In the last few years, membrane chromatography has become a helpful tool for downstream process design. Since the early 2000s and the first FDA approval of membrane use in processes (essentially Q membranes for virus removal), suppliers like Pall, 3M, Millipore or Sartorius have been offering an increasing number of products combining different membrane designs and ligand chemistry.
Why should we use membrane chromatography?
Compared to “classic” column chromatography, using membranes is an asset in terms of:
- Mass transfer: most of the mass transfer is made by convection in a membrane, and it is much more efficient than diffusion mass transfer in traditional resin-bead columns.
- Flow rate: it can be increased significantly, without any negative impact on pressure, so the whole process time decreases. The economic benefit of reducing process time is obvious, especially from the manufacturing point of view. Processing faster can also be a technical advantage during some steps, in terms of stability for example.
- Dealing with large molecules: there can be some issues with proteins with sizes above 200 kDa. With a conventional medium, diffusion through pores can sometimes be difficult and results in a poor yield or very low dynamic binding capacity. Most membrane chromatography media are macroporous and are more suitable to handle these big proteins.
- Easy to handle, single-use: for process development or manufacturing units, setting up a column chromatography step is a routine operation, but when considering column packing time, connections and all cleaning and validation steps, single-use and plug-and-play technologies are so advantageous – nothing new there…
Where do I use my membrane?
Since the very first membranes came on the market, innovation in membrane chromatography has been particularly fast.
Today, membrane chromatography is a well-established technique for polishing (especially Q membranes dedicated to removing HCP and endotoxin and adding some log reduction value (LRV) to your viral clearance). Suppliers have been working on both ligand chemistry but also membrane design so that we now have a wide range of solutions, suitable for almost all process conditions. For example, product concentration or salt levels are no longer obstacles to membrane chromatography.
For a few years, some suppliers have offered membrane chromatography solutions for capture (e.g. Sartorius for protein A), but the technology does not seem to be mature enough to compete with classic media, especially in terms of capacity, and scale-up is still not fully available. Today, the best capture alternative for protein A column, if needed, seems to be ion-exchange or mixed-mode chromatography.
Another interesting aspect is “charged media”, such as 3M Emphaze filters, which are devices combining depth filtration and ion exchange properties and can therefore combine two purification actions in one step. You of course need to remove cells and clarify between USP and DSP, but lowering or eliminating a part of DNA and HCP contaminants before actually starting DSP can be a huge advantage. Even viral clearance efficiency begins to be investigated at this stage.
As always, “the product makes the process”, and when you think about your protein purificationsteps, membrane chromatography is a tool you have to consider. In 15 years, Q membrane has become a gold-standard for the polishing steps, so let’s be prepared for the next step, even if it will be another big culture shift for chromatography!